- Mw Of Water
- Atomic Mass Of Heavy Water
- Zero Mass Stock
- Atomic Weight Of Water
- Atomic Mass Of Water In Grams
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:

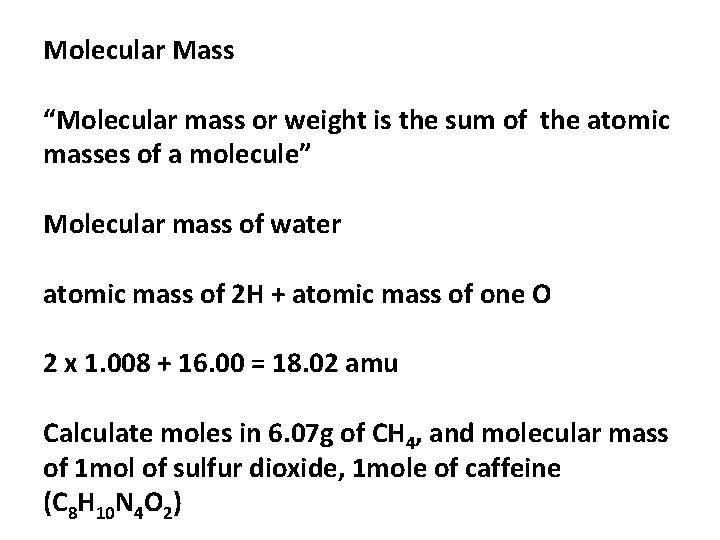
Water (chemical formula: H2O) is a transparent fluid which forms the world's streams, lakes, oceans and rain, and is the major constituent of the fluids of organisms. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a liquid at standard ambient temperature. (a) Atomic mass of hydrogen = 1 u, oxygen = 16 u So the molecular mass of water, which contains two atoms of hydrogen and one atom of oxygen is = 2 × 1 + 1 × 16 = 18u (b) The molecular mass of HNO 3 = the atomic mass of H + the atomic mass of N + 3 × the atomic mass of O = 1 + 14 + 48 = 63 u. Water is not an atom - therefore it does not have an atomic mass. It is a compound so you calculate the molar mass of the compound by adding together the atomic masses of the constituent atoms: H2O.
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
Helo, reders welcome to 'textilesgreen.in' today i m going to explain about molar mass of h2o.
i have made this guide to help you out.

So, hold your seat and be with the end of guide.
So you could get valuable information out of it.
lets get started;
Read More:- Molar mass of CO2?
What is molar mass?
It is define as the mass of sample of that chemical compound divided by the amount of substance in that sample. this phenomenon is called molar mass.
It is measured in moles.
the molar mass of element it can represent gram atomic mass.
the molar mass of compound it can represent gram molecular mass.
If you want to find the Molar mass of chemical compound, such as h2o then you can follow this process.
the relative atomicmass of an element expressed in gram is called gram atomic mass.
For example;
Gram atomic mass of oxygen is = O = 16g
The relative atomic mass of an compound expressed in grams is called gram molecular mass.
For example;
O+O = 16+16 = 32 g
Where, O = oxygen
H+O+H = 1+16+1= 18g
Where, H = Hydrogen and O= oxygen
Read More:- Molar Mass of NaCl?
You can understand easily with the help of following structure.
You know,
1 unit of hydrogen = one atoms of hydrogen.
1 gram of hydrogen = one mole of hydrogen.
Where,
Value of 1 gram of hydrogen is = 6.022×10 rase power of 23.
Similarly,
About H2O (water)
18 units = one molecule of water
18 gram = one moles of water.
Mw Of Water
You know that units is much smaller then grams.

Water (chemical formula: H2O) is a transparent fluid which forms the world's streams, lakes, oceans and rain, and is the major constituent of the fluids of organisms. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a liquid at standard ambient temperature. (a) Atomic mass of hydrogen = 1 u, oxygen = 16 u So the molecular mass of water, which contains two atoms of hydrogen and one atom of oxygen is = 2 × 1 + 1 × 16 = 18u (b) The molecular mass of HNO 3 = the atomic mass of H + the atomic mass of N + 3 × the atomic mass of O = 1 + 14 + 48 = 63 u. Water is not an atom - therefore it does not have an atomic mass. It is a compound so you calculate the molar mass of the compound by adding together the atomic masses of the constituent atoms: H2O.
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
Helo, reders welcome to 'textilesgreen.in' today i m going to explain about molar mass of h2o.
i have made this guide to help you out.
So, hold your seat and be with the end of guide.
So you could get valuable information out of it.
lets get started;
Read More:- Molar mass of CO2?
What is molar mass?
It is define as the mass of sample of that chemical compound divided by the amount of substance in that sample. this phenomenon is called molar mass.
It is measured in moles.
the molar mass of element it can represent gram atomic mass.
the molar mass of compound it can represent gram molecular mass.
If you want to find the Molar mass of chemical compound, such as h2o then you can follow this process.
the relative atomicmass of an element expressed in gram is called gram atomic mass.
For example;
Gram atomic mass of oxygen is = O = 16g
The relative atomic mass of an compound expressed in grams is called gram molecular mass.
For example;
O+O = 16+16 = 32 g
Where, O = oxygen
H+O+H = 1+16+1= 18g
Where, H = Hydrogen and O= oxygen
Read More:- Molar Mass of NaCl?
You can understand easily with the help of following structure.
You know,
1 unit of hydrogen = one atoms of hydrogen.
1 gram of hydrogen = one mole of hydrogen.
Where,
Value of 1 gram of hydrogen is = 6.022×10 rase power of 23.
Similarly,
About H2O (water)
18 units = one molecule of water
18 gram = one moles of water.
Mw Of Water
You know that units is much smaller then grams.
Find the molecular mass of h2o (h2O molar mass)
Find the molecular mass of h2o with the following basic information.
Such as,
First of all, You know that,
atomic mass of hydrogen = 1
atomic mass of oxygen = 16
Where, H= Hydrogen or O = oxygen.
In this chemical compound, it has two atom of Hydrogen and one atom of oxygen is present.
So, two Hydrogen = H2= 1+1 = 2.
Where, atomic mass of hydrogen= 1
But here, two Hydrogen is persent in h2o.
Atomic Mass Of Heavy Water
So, 1+1 = 2 or multiple by 2, in both cases, it will same meaning. and one oxygen is persent in h20.
Zero Mass Stock
it mean, O= oxygen, atomic mass of O = 16
Where,
Atomic mass of oxygen is 16
So, For determine the molar mass of h20 add both atomic mass.
Now, atomic mass of h20 = 1+1+16 = 18 gram
So, molar mass of h2o is = 18 gram.
The molar mass of chemical compound is represente by gram molecular mass.
So, it is represente by 'Gram'
the final result is,
Molar Mass of h2o is 18 gram.
Atomic Weight Of Water
Also read;
• Full form of h2o
Atomic Mass Of Water In Grams
• Nacl full form
